Consider the isotope 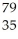
Br.The atomic number is ________,and the mass number is ________.
A) 79;35
B) 35;79
C) 44;35
D) 35;44
E) 35;114
Correct Answer:
Verified
Q27: Atoms of Q28: An isotope with 15 protons and 17 Q29: Adding one proton to the nucleus of Q30: The value of Z for an atom Q31: An atom that contains 47 protons,47 electrons,and Q33: Which elements all belong in the same Q34: Cobalt is element 27.Cobalt-60 is used in Q35: Elements in the Periodic Table are arranged Q36: An imaginary element Xq consists of two Q37: Hydrogen exists as three isotopes.These isotopes differ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents