Which is not a correct statement of Charles's Law?
A) For a gas sample at constant pressure,temperature and volume are inversely proportional.
B) volume α temperature
C) V/T = a constant
D) 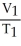
=
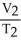
E) none of the above
Correct Answer:
Verified
Q19: Which description best fits a gas?
A)definite shape
Q20: In comparing liquids and gases,liquids have _
Q21: What would be the new pressure if
Q22: Which of the assumptions of the kinetic-molecular
Q23: Which represents the largest pressure?
A)one atmosphere
B)five pounds
Q25: Rank the following in increasing order of
Q26: The barometric pressure given in the local
Q27: Which measurement represents the largest value of
Q28: Using the kinetic molecular theory,as the temperature
Q29: Which is not a correct statement of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents