C5H5N + H2CO3 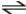 C5H6N ++ HCO3-
C5H6N ++ HCO3-
In the reaction shown,the conjugate acid of C5H5N is
A) C5H5N
B) H2CO3
C) C5H6N +
D) HCO3-.
E) H3O+
Correct Answer:
Verified
Q3: The classification of an acid or a
Q4: Which of the following is a triprotic
Q5: When acids and bases react the product
Q6: A Brønsted-Lowry base is a substance which
A)produces
Q7: Which of the following is a diprotic
Q9: A necessary requirement for a Brønsted base
Q10: What is the conjugate base of HSO4-?
A)SO42-
B)H2SO4
C)H3O+
D)OH-
E)H2SO3
Q11: A Brønsted-Lowry acid is a substance which
A)produces
Q12: Acetic acid is a weak acid in
Q13: According to Brønsted-Lowry theory,acid-base reactions can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents