Osmium has atomic number 76. A particular isotope of osmium has an atomic mass of 191.961 u. Which symbol correctly represents this isotope?
A) 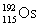
B) 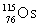
C) 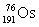
D) 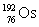
E) 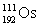
Correct Answer:
Verified
Q12: Which one of the following thicknesses
Q13: The binding energy of an isotope of
Q14: What is the approximate radius of a
Q15: How many neutrons are there in the
Q16: Which one of the following types
Q18: Radiation or(and) particles emerge(s) from a
Q19: Which entry in the table below
Q20: What is the SI unit for activity?
A)Ci
B)counts/min
C)Hz
D)Gy
E)Bq
Q21: Which process is involved in determining the
Q22: The activity of carbon-14 in a sample
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents