The same activity is measured for two different isotope samples. One sample contains 0.0450 kg of 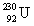 (atomic mass = 230.033 937 u, t1/2 = 20.8 days) . The second sample contains an unknown amount of
(atomic mass = 230.033 937 u, t1/2 = 20.8 days) . The second sample contains an unknown amount of 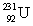 (atomic mass = 231.036 264 u, t1/2 = 4.3 days) . What is the mass of the second sample?
(atomic mass = 231.036 264 u, t1/2 = 4.3 days) . What is the mass of the second sample?
A) 0.0093 kg
B) 0.23 kg
C) 0.110 kg
D) 0.037 kg
E) 0.0450 kg
Correct Answer:
Verified
Q20: What is the SI unit for activity?
A)Ci
B)counts/min
C)Hz
D)Gy
E)Bq
Q21: Which process is involved in determining the
Q22: The activity of carbon-14 in a sample
Q23: Determine the activity of 6.0 × 1012
Q24: Which one of the following statements
Q26: If the uranium nucleus is at rest
Q27: An isotope of krypton has a half-life
Q28: The ratio of the abundance of carbon-14
Q29: Tritium is an isotope of hydrogen
Q30: What is X?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents