An electron in an atom has the following set of quantum numbers:
n = 2, 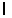 = 1,
= 1, 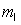 = -1, ms = +1/2.
= -1, ms = +1/2.
-What shell is this electron occupying?
A) K shell
B) L shell
C) M shell
D) N shell
E) O shell
Correct Answer:
Verified
Q49: Electrons in an X-ray tube are accelerated
Q50: Consider the following list of electron configurations:
Q51: Which one of the following statements concerning
Q52: What is the operating voltage of a
Q53: A neutral atom has the following electronic
Q55: An electron in an atom has the
Q56: Consider the following list of electron configurations:
Q57: Which electron energy will produce the largest
Q58: Use the Bohr model to estimate
Q59: The ground state electronic configuration of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents