A mass spectrometer is used to separate two isotopes of uranium with masses m1 and m2 where m2 > m1. The two types of uranium atom exit an ion source S with the same charge of +e and are accelerated through a potential difference V. The charged atoms then enter a constant, uniform magnetic field B as shown. If r1 = 0.5049 m and r2 = 0.5081 m, what is the value of the ratio m1/m2? 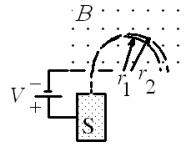
A) 0.9984
B) 0.9937
C) 0.9874
D) 0.9812
E) 0.9749
Correct Answer:
Verified
Q7: A charged particle is moving in a
Q8: An electron traveling due north enters a
Q9: Two particles move through a uniform magnetic
Q10: An electron is moving with a speed
Q11: Two electrons are located in a region
Q13: A particle with a mass of 6.64
Q14: A proton traveling due west in a
Q15: Complete the following statement: The magnitude of
Q16: Which one of the following statements concerning
Q17: Two charged particles of equal mass are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents