An ideal gas is taken from state A to state B through process shown on the pressure-volume graph. How much heat is added to the gas in this process? 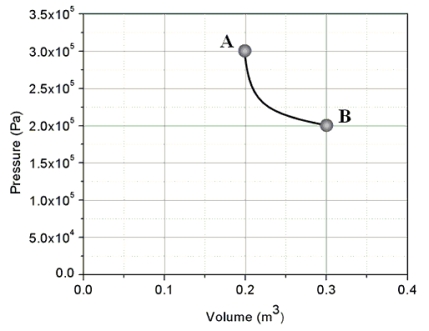
A) zero joules
B) 1.0 × 104 J
C) 2.4 × 104 J
D) 6.0 × 104 J
E) This cannot be determined since n and T are not specified.
Correct Answer:
Verified
Q15: Complete the following statement: Walls that separate
Q19: 5.00 kg of liquid water is heated
Q20: Which one of the following situations is
Q21: An ideal monatomic gas expands isobarically from
Q22: Beneath the moveable piston in the drawing,2.25
Q24: A quantity of carbon monoxide gas is
Q25: A paddle wheel frictionally adds thermal energy
Q30: One mole of a monatomic gas at
Q35: An ideal monatomic gas originally in state
Q40: During one stage of a reversible process,the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents