An ideal monatomic gas expands isothermally from state A to state B. The gas then cools at constant volume to state C. The gas is then compressed isobarically to D before it is heated until it returns to state A. 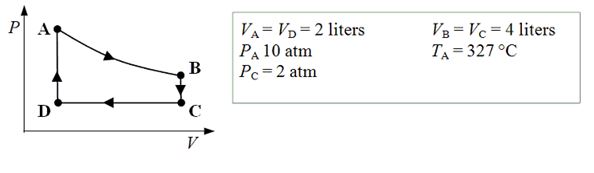
-What is the internal energy of the gas at point B?
A) 1 × 103 J
B) 2 × 103 J
C) 3 × 103 J
D) 4 × 103 J
E) 5 × 103 J
Correct Answer:
Verified
Q46: A Carnot engine operates between hot and
Q47: Which one of the following statements is
Q54: What is the maximum possible efficiency of
Q55: Under which one of the following conditions
Q57: The ratio of the molar specific
Q65: A block that slides on a rough
Q66: In an isothermal and reversible process, 945
Q68: A container holding 1.2 kg of water
Q71: Which one of the following processes represents
Q73: An ideal monatomic gas expands isothermally from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents