A 200.0-kg object is attached via an ideal pulley system to paddle wheels that are submerged in 0.480 kg of glycerin at 20.0 °C in an insulated container as shown. Then, the object falls through a distance of 5.00 m causing the paddle wheel to turn. Assuming all of the mechanical energy lost by the falling object goes into the water, determine the final temperature of the glycerin. The specific heat capacity of glycerin is 2410 J/ 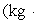 Co) .
Co) . 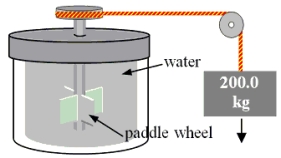
A) 4.90 °C
B) 28.5 °C
C) 24.9 °C
D) 40.4 °C
E) 8.47 °C
Correct Answer:
Verified
Q22: Complete the following statement: The term heat
Q23: Complete the following statement: When a substance
Q24: Heat is added to a substance, but
Q25: A 2.00-kg metal object requires 1.00 ×
Q26: A gold sphere has a radius of
Q28: Which one of the following phrases is
Q29: The coefficient of volumetric expansion for gold
Q30: Two cubes, one silver and one iron,
Q31: A 2.0-g sample of steam at 100
Q32: Complete the following statement: When solid NH3
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents