A 0.0500-kg lead bullet of volume 5.00 × 10–6 m3 at 20.0 °C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At that time, the temperature of the bullet is 327 °C. Use the following information for lead: 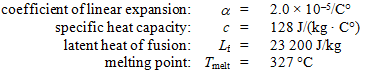
-How much heat was needed to raise the bullet to its final temperature?
A) 963 J
B) 1960 J
C) 3640 J
D) 3880 J
E) 4440 J
Correct Answer:
Verified
Q50: A 0.040-kg ice cube at 0 °C
Q51: Determine the latent heat of vaporization of
Q52: Using the data in the table, determine
Q53: A thermos bottle contains 3.0 kg of
Q54: Heat is added to a sample of
Q56: In an insulated container, 0.50 kg of
Q57: Given the following information, determine the relative
Q58: Ryan places 0.150 kg of boiling water
Q59: Which would cause a more serious burn:
Q60: A 0.030-kg ice cube at 0 °C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents