A 0.0500-kg lead bullet of volume 5.00 × 10–6 m3 at 20.0 °C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At that time, the temperature of the bullet is 327 °C. Use the following information for lead: 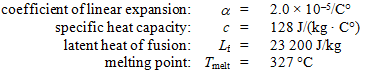
-What additional heat would be needed to melt the bullet?
A) 420 J
B) 628 J
C) 837 J
D) 1160 J
E) 2010 J
Correct Answer:
Verified
Q42: A 0.0500-kg lead bullet of volume 5.00
Q43: A household humidifier continuously takes water in
Q44: What is the minimum amount of energy
Q45: An ordinary mercury thermometer at room temperature
Q46: A liquid is in equilibrium with its
Q48: The graph shows the equilibrium vapor pressure
Q49: On a warm summer day, the relative
Q50: A 0.040-kg ice cube at 0 °C
Q51: Determine the latent heat of vaporization of
Q52: Using the data in the table, determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents