Given the following standard reaction enthalpies: 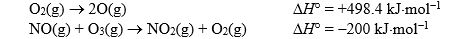 And the standard enthalpy of formation of ozone,+142.7 kJ.mol - 1,calculate the standard reaction enthalpy for the reaction
And the standard enthalpy of formation of ozone,+142.7 kJ.mol - 1,calculate the standard reaction enthalpy for the reaction
NO(g) + O(g) NO2(g)
A) (-306 kJ.mol - 1 )
B) +355 kJ.mol - 1
C) +592 kJ.mol - 1
D) +555 kJ.mol - 1
E) +192 kJ.mol - 1
Correct Answer:
Verified
Q81: What is the standard enthalpy of formation
Q85: In the combustion of coal,C(s),the C=O bonds
Q86: What type of process is the formation
Q86: For gaseous hydrogen atoms at 298
Q88: When nitrogen gas expands from 3.00
Q89: Calculate the standard reaction enthalpy for the
Q91: for the reaction
N2O5(s)
Q92: Of the following, which is not a
Q93: All bond enthalpies are positive.
Q100: What can an isolated system exchange with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents