Consider the following compounds and their standard free energies of formation: 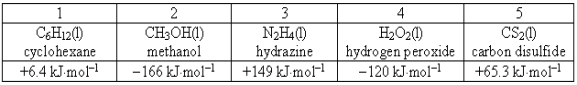 Which of these liquids is (are) thermodynamically stable?
Which of these liquids is (are) thermodynamically stable?
A) 2 and 4
B) 2 and 3
C) 1, 3, and 5
D) 1
E) 3
Correct Answer:
Verified
Q55: Calculate
Q124: The reaction N2(g)+3H2(g)
Q131: Consider the reaction
2SO3(g)
Q137: The reaction 2C(s)+ 2H2(g)
Q142: Under what conditions (e.g.,constant P)are the
Q146: For the reaction
2C(s)+ 2H2(g)
Q154: Which of the following statements is
Q155: The reaction CH3CH2CH2CH3(g)
Q159: Consider the reaction
Cl2(g)
Q160: For the reaction
2C(s)+ 2H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents