The phase diagram for a pure substance is shown below. 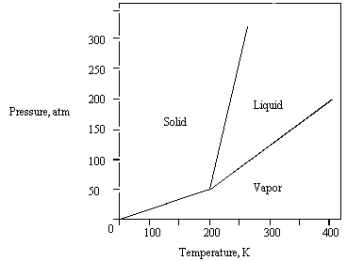 At 100 atm and 250 K,the substance exists as
At 100 atm and 250 K,the substance exists as
A) both vapor and liquid in equilibrium.
B) liquid.
C) both vapor and solid in equilibrium.
D) vapor.
E) solid.
Correct Answer:
Verified
Q23: For a one-component system, at the triple
Q23: When three phases are in mutual equilibrium,such
Q30: The phase diagram for a pure substance
Q31: The phase diagram for CO2 is
Q33: The phase diagram for carbon dioxide
Q35: The phase diagram for sulfur is given
Q36: Consider the phase diagrams for water and
Q37: A gas is a form of matter
Q38: The phase diagram for a pure substance
Q39: What is the highest temperature that the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents