The phase diagram for a pure substance is given below.The solid sublimes 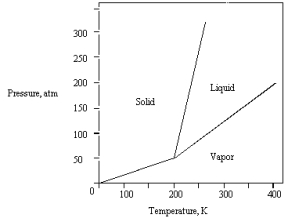
A) at 400 K and 200 atm.
B) at 200 K and 100 atm.
C) at 300 K and 100 atm.
D) at 300 K and 75 atm.
E) if warmed at any pressure below 50 atm.
Correct Answer:
Verified
Q2: Estimate the enthalpy of vaporization of
Q12: Why is the vapor pressure of cis-dibromoethene
Q18: Which of the following liquids freeze at
Q20: the boiling point of water is
Q21: The phase diagram for sulfur is given
Q21: the triple point for water,4.6
Q25: The phase diagram for a pure substance
Q26: The phase diagram for sulfur is given
Q30: Consider the phase diagrams for water and
Q32: Consider the phase diagram for sulfur in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents