Consider the diagram below. 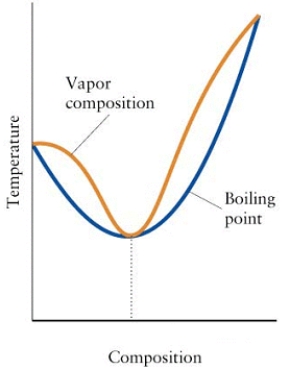 (a)What is the mixture in the diagram called?
(a)What is the mixture in the diagram called?
(b)Can the components of this mixture be separated by fractional distillation? Explain.
Correct Answer:
Verified
(...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: For AlF3,the lattice enthalpy is 5220
Q44: Aqueous ammonia (28%)is 15.0 M and has
Q46: What is the molality of carbon tetrachloride
Q47: Benzene would likely dissolve which of the
Q61: the van't Hoff i of HF differs
Q62: The osmotic pressure of 1.00 g
Q65: Which of the following pairs have a
Q70: The addition of 125 mg of caffeine
Q78: The normal boiling point of ethanol
Q80: Which of the following 1.0 M solutions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents