The following phase diagram is for a pure substance. 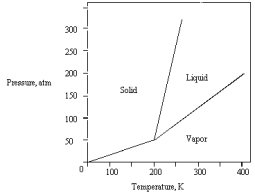 Below what temperature does the substance exist as a liquid?
Below what temperature does the substance exist as a liquid?
A) 150 K
B) 200 K
C) 300 K
D) 350 K
E) 400 K
Correct Answer:
Verified
Q67: Water and acetone,CH3COCH3,both freeze at a higher
Q69: The critical temperature of N2 is
Q73: Which of the following pairs have a
Q76: Which of the following has the lowest
Q85: On what conditions does the location
Q88: Using a mass of 500.0 g of
Q92: With which of the following solutes can
Q93: the van't Hoff i of HBr,HCl,and HF
Q95: Which of the following has the smallest
Q98: Why is the vapor pressure of trans-dibromoethene
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents