Multiple Choice
Given: SO2(g) 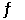 O2(g) + S(s)
O2(g) + S(s)
Kc = 2.5 *10-53
SO3(g) 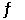
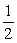 O2(g) + SO2(g)
O2(g) + SO2(g)
Kc = 4.0 *10-13
Calculate Kc for the reaction
2S(s) + 3O2(g) 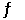 2SO3(g)
2SO3(g)
A) 1.6 *10103
B) 1.6 *1080
C) 1.0 * 10130
D) 1.6 * 1040
E) 1.0 *1065
Correct Answer:
Verified
Related Questions
Q23: Given: C(s)+ CO2(g) Q24: Given: 2SO2(g)+ O2(g) Q25: The equilibrium constant,K,for the reaction 2HgO(s) Q26: What is the relationship between K and Q27: At 700 K,K = 54 for the Q29: Given: 4NH3(g)+ 5O2(g) Q30: Given: 2SO2(g)+ O2(g) Q31: At 25 Q32: Given: 4NH3(g)+ 5O2(g) Q33: At 25 Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()

