The following compounds are available as 0.10 M aqueous solutions. 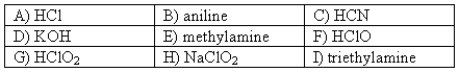 Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
Correct Answer:
Verified
Q24: Calculate the ratio of the molarities of
Q28: The following compounds are available as 0.10
Q29: The following compounds are available as 0.10
Q30: For NH3,pKb = 4.74.What is the pH
Q35: If a small amount of a strong
Q35: The following compounds are available as 0.10
Q37: If a small amount of a strong
Q38: Calculate the ratio of the molarities of
Q120: Which of the following mixtures gives a
Q124: A buffer solution contains 0.25 M NaNO2(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents