The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq) is: 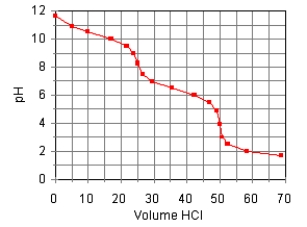 Estimate pKb1.
Estimate pKb1.
A) 8.5
B) 6.4
C) 3.7
D) 10.3
E) 7.6
Correct Answer:
Verified
Q71: The Cu2+ ion can be separated from
Q75: What is the relationship between the solubility
Q78: The titration curve for the titration of
Q79: If you wish to increase the solubility
Q156: A certain weak acid has a Ka
Q158: What is the equilibrium constant for the
Q165: What is the main factor that determines
Q170: If the molar solubility of the compound
Q183: In the titration of 0.300 M CH3NH2(aq)with
Q193: How many moles of KOH(s)must be added
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents