Calculate the value of the equilibrium constant for the reaction AgCl(s) + 2NH3(aq) 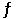 Ag(NH3) 2+(aq) + Cl - (aq)
Ag(NH3) 2+(aq) + Cl - (aq)
Given Ksp = 1.6*10 - 10 for silver chloride and Kf = 1.6 *107 for the ammonia complex of Ag+ ions,Ag(NH3) 2+.
A) 1.0 *10 - 17
B) 6.3 *109
C) 6.3 *10 - 8
D) 1.0 * 1017
E) 2.6 *10 - 3
Correct Answer:
Verified
Q61: The titration curve for the titration of
Q65: Consider the titration of 15.0 mL of
Q68: The titration curve for the titration of
Q70: If equal volumes of 0.004 M Pb(NO3)2(aq)and
Q70: You have available the following reagents as
Q162: Consider the titration of 15.0 mL of
Q168: Silver bromide is most soluble in
A) pure
Q176: What is the equilibrium constant for the
Q178: Calculate the solubility product of calcium
Q180: Which of the following water-insoluble salts is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents