What is the equilibrium constant for the reaction HCN(aq) + OH-(aq) 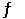 CN-(aq) + H2O(l)
CN-(aq) + H2O(l)
A) Ka/Kw
B) Kb
C) Kw/Ka
D) Kw/Kb
E) Kb/Kw
Correct Answer:
Verified
Q83: If 10.0 mmol of sodium hydroxide is
Q86: The weak base,B,has Kb = 3.1 ×
Q87: You have available the following reagents as
Q94: The Ksp for mercury(I)iodide is 1.2 *
Q164: When silver ions form a coordination complex
Q173: The solubility of silver bromide is greater
Q179: The solubility of silver chloride is greater
Q182: In the titration of 0.300 M H3PO4(aq)with
Q192: If 50.0 mL of 0.22 M NaCl(aq)is
Q196: Assuming no volume change on mixing,what mass
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents