Use the following to answer questions 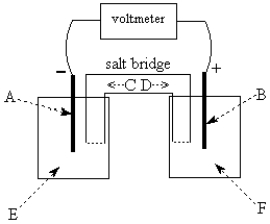
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or lead.
Correct Answer:
Verified
Q44: The standard voltage of the cell
Q47: If E
Q48: Use the following diagram of a
Q50: The standard voltage of the cell
Q51: The standard voltage of the cell
Q52: Consider the following cell: Zn(s)|Zn2+(aq,0.100 M)m
Q221: Calculate E for the half-reaction below.
2H+(aq,1.00
Q231: The equilibrium constant for the reaction
2Hg(l)+
Q238: Consider the following cell:
Ag(s)|Ag+(aq,0.100 M)mAg+(aq,0.100 M)|Ag(s)
What is
Q245: Use the following to answer questions 55-58:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents