The phase diagram for a pure substance is given below. 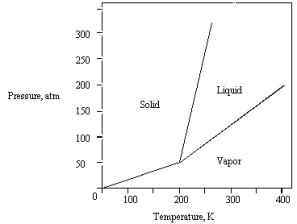 The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.
The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.
A) The liquid in the container freezes.
B) The solid in the container sublimes.
C) The solid in the container melts.
D) The vapor in the container escapes.
E) The liquid in the container vaporizes.
Correct Answer:
Verified
Q17: The vapor pressure of water at
Q18: Which of the following liquids freeze at
Q19: The phase diagram for a pure compound
Q20: the boiling point of water is
Q21: the triple point for water,4.6
Q23: For a one-component system, at the triple
Q24: The phase diagram for CO2 is given
Q25: The phase diagram for a pure substance
Q26: The phase diagram for a pure substance
Q27: What is the highest temperature that the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents