For the Equilibrium CaCO3(s) CaO(s)+ CO2(g),
(A)represents the Composition at Equilibrium at a Certain
For the equilibrium CaCO3(s) CaO(s)+ CO2(g),
(a)represents the composition at equilibrium at a certain temperature.In (b),a small amount of Ca*CO3(s) has been added (Ca*CO3(s)represents Ca14CO3(s)or labeled calcium carbonate).Draw the composition in (c) at equilibrium and explain your drawing. 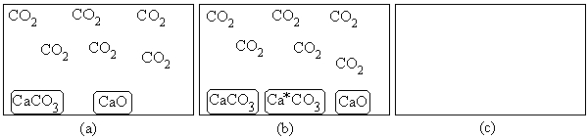
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q84: If a 1-L flask containing D2(g),N2(g),and ND3(g)at
Q85: On what conditions does the location
Q86: Of the following, which would likely dissolve
Q87: The increase in entropy of the system
Q88: Using a mass of 500.0 g of
Q90: Consider the following reaction at a
Q91: The lattice enthalpy and the enthalpy of
Q92: With which of the following solutes can
Q93: the van't Hoff i of HBr,HCl,and HF
Q94: What happens to the boiling point of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents