The fractional composition diagram for the amino acid alanine is given below. 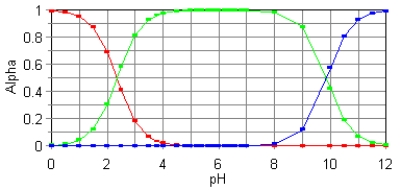
Write the structure of the dominant species at pH 1,6,and 12,respectively.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q58: The Ka of phenol is 1.3 *
Q59: For a solution labeled "0.10 M H3PO4(aq),"
A)
Q60: The Ka of phenol is 1.3 *
Q61: The amino acid alanine,HOOC-CH(CH3)NH3+,Has Ka1 =
Q62: The boxes below contain a series
Q64: The pH of 0.010 M H3PO4(aq) is
Q65: Of the following, which is not a
Q66: The amino acid alanine, HOOC-CH(CH3)NH3+,Has Ka1
Q67: Calculate the equilibrium constant for the
Q68: If
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents