The titration curve for the titration of 0.100 M H2SO3(aq) with 0.100 M KOH(aq) Is given below. 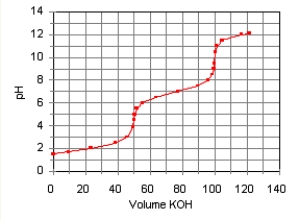
The major species in solution after 75 mL of KOH(aq) Has been added are
A) HSO3-(aq) and Na+(aq) .
B) SO32-(aq) , and Na+(aq) .
C) SO32-(aq) , OH-(aq) , and Na+(aq) .
D) H2SO3(aq) , HSO3-, and Na+(aq) .
E) HSO3-(aq) , SO32-(aq) , and Na+(aq) .
Correct Answer:
Verified
Q141: What is the pH at the half-stoichiometric
Q142: What is the pH at the half-stoichiometric
Q143: Which of the following indicators would be
Q144: Consider the titration of 50.0 mL of
Q145: What is the concentration of acetate ion
Q147: Which of the following indicators would be
Q148: The titration curve for the titration of
Q149: The titration curve for the titration of
Q150: What is the pH at the stoichiometric
Q151: What is the concentration of acetate ion
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents