Use the following to answer questions 55-58: 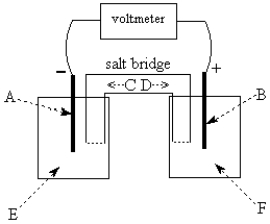
-The galvanic cell shown above uses the half-cells Mg2+/Mg and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q255: Use the following diagram of a
Q256: Sodium is produced by electrolysis of molten
Q257: Use the following diagram of a
Q258: When a lead-acid battery discharges,sulfuric acid is
Q259: Use the following diagram of a
Q261: What is E for the half-reaction
Q262: For the cell diagram
Pt|H2(g)|H+(aq)mCo3+(aq),Co2+(aq)|Pt
write the reaction that
Q263: Consider the following cell:
Zn(s)|Zn2+(aq,0.10 M)m Cu2+(aq,0.10 M)|Cu(s)
At
Q264: What are the products of the electrolytic
Q265: Consider the following cell:
Zn(s)|Zn2+(aq,0.10 M)m Cu2+(aq,0.10 M)|Cu(s)
At
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents