Which combination of protons,neutrons,and electrons is correct for the isotope of copper, 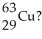
A) 29 p+, 34 n°, 29 e-
B) 29 p+, 29 n°, 63 e-
C) 63 p+, 29 n°, 63 e-
D) 34 p+, 29 n°, 34 e-
E) 34 p+, 34 n°, 29 e-
Correct Answer:
Verified
Q25: The atomic mass unit is presently based
Q26: In the symbol below,X = _.
Q27: In the symbol below,x is _.
Q28: Gravitational forces act between objects in proportion
Q29: Which one of the following basic forces
Q31: Which isotope has 36 electrons in an
Q32: Which isotope has 45 neutrons?
A) 
Q33: Different isotopes of a particular element contain
Q34: Which of the following atoms has the
Q35: In the symbol below,x = _.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents