Silver has two naturally occurring isotopes with the following isotopic masses: 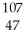 Ar
Ar 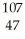 Ar 106.90509 108.9047
Ar 106.90509 108.9047
The average atomic mass of silver is 107.8682 amu.The fractional abundance of the lighter of the two isotopes is ________.
A) 0.24221
B) 0.48168
C) 0.51835
D) 0.75783
E) 0.90474
Correct Answer:
Verified
Q17: Of the three types of radioactivity characterized
Q18: Of the three types of radioactivity characterized
Q19: A molecule of water contains hydrogen and
Q20: Of the three types of radioactivity characterized
Q21: Which pair of atoms constitutes a pair
Q23: The nucleus of an atom does not
Q24: Different isotopes of a particular element contain
Q25: The atomic mass unit is presently based
Q26: In the symbol below,X = _.
Q27: In the symbol below,x is _.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents