The element X has three naturally occurring isotopes.The masses (amu) and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 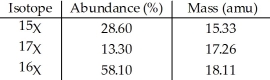
A) 17.20
B) 16.90
C) 17.65
D) 17.11
E) 16. 90
Correct Answer:
Verified
Q46: Vanadium has two naturally occurring isotopes,50V with
Q47: An element that appears in the lower
Q48: An unknown element is found to have
Q49: An element in the upper right corner
Q50: Elements in the same group of the
Q52: Which pair of elements would you expect
Q53: Elements _ exhibit similar physical and chemical
Q54: The average atomic weight of copper,which has
Q55: The element X has three naturally occurring
Q56: The element X has two naturally occurring
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents