The value of ΔH° for the reaction below is -6535 kJ. ________ kJ of heat are released in the combustion of 16.0 g of 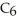
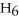 (l) ?
(l) ?
2C6H6 (l) + 15O2 (g) → 12CO2 (g) + 6H2O (l)
A) 1.34 × 103
B) 5.23 × 104
C) 669
D) 2.68 × 103
E) -6535
Correct Answer:
Verified
Q42: The kinetic energy of a 23.2-g object
Q43: What is the kinetic energy of a
Q44: Carbon monoxide and oxygen gas react to
Q45: Calculate the kinetic energy in joules of
Q46: The kinetic energy of a 7.3 kg
Q48: Calculate the kinetic energy in joules of
Q49: Calculate the kinetic energy in joules of
Q50: The ΔE of a system that absorbs
Q51: The change in the internal energy of
Q52: A 100-watt electric incandescent light bulb consumes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents