The specific heat capacity of lead is 0.13 J/g-K.How much heat (in J) is required to raise the temperature of 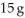 of lead from 22 °C to 37 °C?
of lead from 22 °C to 37 °C?
A) 2.0 J
B) -0.13 J
C) 5.8 × 10-4 J
D) 29 J
E) 0.13 J
Correct Answer:
Verified
Q74: Given the following reactions N2 (g)+ 2O2
Q75: Given the following reactions 2NO → N2
Q76: What is the molar heat capacity (in
Q77: The value of ΔH° for the reaction
Q78: The value of ΔH° for the reaction
Q80: The value of ΔH° for the reaction
Q81: Given the following reactions N2 (g)+ O2
Q82: Given the data in the table below,ΔH°rxn
Q83: Given the data in the table below,ΔH°rxn
Q84: Given the data in the table below,ΔH°
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents