Given the following reactions N2 (g) + O2 (g) → 2NO (g) ΔH = +180.7 kJ
2NO (g) + O2(g) → 2N 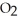 (g) ΔH = -113.1 kJ
(g) ΔH = -113.1 kJ
The enthalpy of reaction for
4NO (g) → 2NO2 (g) + N2 (g)
Is ________ kJ.
A) 67.6
B) 45.5
C) -293.8
D) -45.5
E) 293.8
Correct Answer:
Verified
Q84: Given the data in the table below,ΔH°
Q85: Given the data in the table below,ΔH°rxn
Q86: The value of ΔH° for the reaction
Q87: Given the data in the table below,ΔH°rxn
Q88: The value of ΔH° for the following
Q90: Given the data in the table below,ΔH°rxn
Q91: Given the data in the table below,ΔH°rxn
Q92: Given the following reactions H2O (l)→ H2O
Q93: Given the following reactions N2 (g)+ O2
Q94: Given the data in the table below,ΔH°rxn
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents