The specific heat capacity of liquid water is 4.18 J/g-K.How many joules of heat are needed to raise the temperature of 7.25 g of water from 20.0 °C to 44.1 °C?
A) 41.8 J
B) 730 J
C) 1.94 × 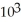 J
J
D) 2.39 × 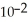 J
J
E) 66.8 J
Correct Answer:
Verified
Q121: The temperature of a 35.1 g sample
Q122: What is the enthalpy change (in kJ)of
Q123: In the presence of excess oxygen,methane gas
Q124: The temperature of a 24.3 g sample
Q125: A 10.1 g sample of NaOH is
Q127: The specific heat capacity of liquid mercury
Q128: An 6.11 g sample of calcium carbonate
Q129: A sample of iron absorbs 81.0 J
Q130: How many joules of heat are absorbed
Q131: A sample of calcium carbonate [CaCO3 (s)]
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents