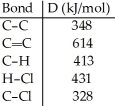
Using the table of bond dissociation energies,the ΔH for the following gas-phase reaction is ________ kJ. 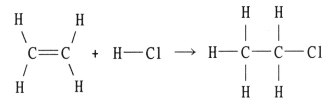
A) -44
B) 38
C) 304
D) 2134
E) -38
Correct Answer:
Verified
Q77: The ability of an atom in a
Q78: The ion NO- has _ valence electrons.
A)15
B)14
C)16
D)10
E)12
Q79: A triple bond consists of _ pairs
Q80: Determining lattice energy from Born-Haber cycle data
Q81: How many equivalent resonance structures can be
Q83: Using the table of average bond energies
Q84: Using the table of bond dissociation energies,the
Q85: In the Lewis structure of HCO3-,the formal
Q86: The formal charge on sulfur in SO42-
Q87: In the Lewis structure of ClF,the formal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents