The molecular geometry of the left-most carbon atom in the molecule below is ________. 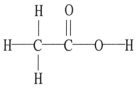
A) trigonal planar
B) trigonal bipyramidal
C) tetrahedral
D) octahedral
E) T-shaped
Correct Answer:
Verified
Q15: The electron-domain geometry of _ is tetrahedral.
A)CBr4
B)PH3
C)CCl2Br2
D)XeF4
E)all
Q16: Of the molecules below,only _ is polar.
A)CCl4
B)CH4
C)SeF4
D)SiCl4
Q17: The bond angles marked a,b,and c in
Q18: PCl5 has _ electron domains and a
Q19: In counting the electron domains around the
Q21: The hybrid orbitals used for bonding by
Q22: For molecules with only one central atom,how
Q23: The molecular geometry of the CHF3 molecule
Q24: The sp3d2 atomic hybrid orbital set accommodates
Q25: What are the hybrid orbitals used for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents