Of the following molecules,only ________ is polar.
A) BeCl2
B) BF3
C) C 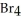
D) SiH2Cl2
E) Cl2
Correct Answer:
Verified
Q27: Which of the molecules has a square
Q28: Of the following molecules,only _ is polar.
A)CCl4
B)BCl3
C)NCl3
D)BeCl2
E)Cl2
Q29: The molecular geometry of the PF3 molecule
Q30: The hybridizations of nitrogen in NF3 and
Q31: The sp2 atomic hybrid orbital set accommodates
Q33: Which of the molecules has a see-saw
Q34: Three monosulfur fluorides are observed: SF2,SF4,and SF6.Of
Q35: The hybridizations of iodine in IF3 and
Q36: Of the following,the central atom is sp3d2
Q37: The molecular geometry of the BCl3 molecule
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents