The order of MO energies in B2,C2,and N2 (σ2p > π2p) ,is different from the order in O2,F2,and Ne2 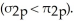 This is due to ________.
This is due to ________.
A) less effective overlap of p orbitals in O2, F2, and Ne2
B) the more metallic character of boron, carbon and nitrogen as compared to oxygen, fluorine, and neon
C) greater 2s-2p interaction in O2, F2, and Ne2
D) greater 2s-2p interaction in B2, C2, and N2
E) less effective overlap of p orbitals in B2, C2, and N2
Correct Answer:
Verified
Q79: In comparing the same two atoms bonded
Q80: According to molecular orbital theory,the bond order
Q81: For a molecule with the formula AB3,the
Q82: The more effectively two atomic orbitals overlap,_.
A)the
Q83: An antibonding MO _ the corresponding bonding
Q85: The molecular geometry of the CS2 molecule
Q86: The molecular geometry of _ is square
Q87: The F-B-F bond angle in the BF2-
Q88: The F-B-F bond angle in the BF3
Q89: The molecular geometry of the SF2 molecule
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents