There is/are ________ π bond(s) in the molecule below. 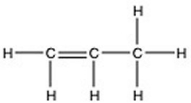
A) 7
B) 6
C) 2
D) 1
E) 0
Correct Answer:
Verified
Q111: The electron-domain geometry of a sulfur-centered compound
Q112: The angles between sp2 orbitals are _.
A)45°
B)180°
C)90°
D)109.5°
E)120°
Q113: In order to produce sp3 hybrid orbitals,_
Q114: The hybridization of orbitals on the central
Q115: The Lewis structure of carbon monoxide is
Q117: The hybridization of the central atom in
Q118: The hybridization of orbitals on the central
Q119: There are _ σ and _ π
Q120: In order to produce sp2 hybrid orbitals,_
Q121: The electron-domain geometry and molecular geometry of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents