In ideal gas equation calculations,expressing pressure in Pascals (Pa) ,necessitates the use of the gas constant,R,equal to ________.
A) 0.08206 atm L mol-1K-1
B) 8.314 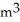 -Pa/mol-K
-Pa/mol-K
C) 62.36 L torr mol-1K-1
D) 1.987 cal mol-1K-1
E) none of the above
Correct Answer:
Verified
Q4: Which one of the following is a
Q5: Which of the following equations shows an
Q6: Standard temperature and pressure (STP),in the context
Q7: Which of the following is not a
Q8: Of the following,_ has the odor of
Q10: The molar volume of a gas at
Q11: Of the following,_ has a strong acrid
Q12: "Isothermal" means _.
A)at constant pressure
B)at constant temperature
C)at
Q13: The pressure exerted by a column of
Q14: Gaseous mixtures _.
A)can only contain molecules
B)are all
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents