A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 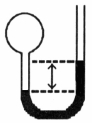 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
A) 1.05
B) 1.01
C) 0.976
D) 0.993
E) 1.08
Correct Answer:
Verified
Q53: Which one of the following gases would
Q54: A sample of a gas originally at
Q55: Two gases start to escape from a
Q56: If 50.75 g of a gas occupies
Q57: A gas vessel is attached to an
Q59: Which noble gas is expected to show
Q60: How much faster does 79Br2 effuse than
Q61: What is the molecular weight (g/mol)of an
Q62: If pressure and temperature are kept constant,the
Q63: The reaction of 50 mL of Cl2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents