Based on molecular mass and dipole moment of the five compounds in the table below,which should have the highest boiling point? 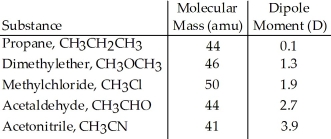
A) CH3CH2CH3
B) CH3OCH3
C) CH3Cl
D) CH3CHO
E) CH3CN
Correct Answer:
Verified
Q62: The heating curve shown was generated by
Q63: Molecules with _ do not generally exhibit
Q64: In the _ liquid crystalline phase,the component
Q65: The phase diagram of a substance is
Q66: The heating curve shown was generated by
Q68: On the phase diagram shown above,segment _
Q69: On the phase diagram shown above,the coordinates
Q70: For a given substance that exhibits liquid-crystalline
Q71: _ liquid crystals are colored because the
Q72: The phase diagram of a substance is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents