The heat of fusion of water is 6.01 kJ/mol.The heat capacity of liquid water is 75.3 J/mol ∙ K.The conversion of 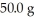 of ice at 0.00 °C to liquid water at 0.00°C requires ________ kJ of heat.
of ice at 0.00 °C to liquid water at 0.00°C requires ________ kJ of heat.
A) 6.01
B) 16.7
C) 75.3
D) 17.2
E) Insufficient data are given.
Correct Answer:
Verified
Q99: According to the phase diagram shown above,what
Q100: What is the predominant intramolecular force in
Q101: In general,intramolecular forces determine the _ properties
Q102: Based on the figure above,what is the
Q103: The phase diagram of a substance is
Q105: Of the following,_ is the most volatile.
A)C2H6
B)C2I6
C)C2Br6
D)C2Cl6
E)C2F6
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents