Ethanol ( 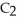
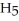 OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
A) 207.3
B) -13.3
C) 6.34
D) 3617
E) 8.63
Correct Answer:
Verified
Q101: In general,intramolecular forces determine the _ properties
Q102: Based on the figure above,what is the
Q103: The phase diagram of a substance is
Q104: The heat of fusion of water is
Q105: Of the following,_ is the most volatile.
A)C2H6
B)C2I6
C)C2Br6
D)C2Cl6
E)C2F6
Q107: The enthalpy change for converting 10.0 g
Q108: The principal source of the difference in
Q109: Based on the figure above,the boiling point
Q110: The conversion of a solid to a
Q111: London Dispersion Forces tend to _ in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents