Based on the figure above,the boiling point of diethyl ether under an external pressure of 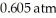 is ________ °C.
is ________ °C.
A) 40
B) 10
C) 30
D) 20
E) 0
Correct Answer:
Verified
Q111: London Dispersion Forces tend to _ in
Q112: The enthalpy change for converting 1.00 mol
Q113: Calculate the enthalpy change (in kJ)associated with
Q114: What is the predominant intermolecular force in
Q115: The initial discovery of a liquid crystal
Q117: What is the predominant intermolecular force in
Q118: Ethanol melts at -114 °C and boils
Q119: At 1 atm,an unknown sample melts at
Q120: Which molecule is the least volatile?
A)CH3Cl
B)CH3I
C)CH3F
D)CH4
E)CH3Br
Q121: The boiling points of normal hydrocarbons are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents