A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water.The density of the resulting solution is 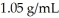 .The concentration of Cl- in this solution is ________ M.
.The concentration of Cl- in this solution is ________ M.
A) 0.214
B) 0.562
C) 1.12
D) 1.20
E) 6.64 × 10-2
Correct Answer:
Verified
Q73: A solution is prepared by dissolving 15.8
Q74: The solubility of MnSO4 monohydrate in water
Q75: The vapor pressure of pure ethanol at
Q76: What is the mole fraction of HCl
Q77: A solution is prepared by dissolving 15.0
Q79: What is the molality of LiCl in
Q80: A solution is prepared by dissolving 25.0
Q81: A solution is prepared by dissolving 27.7
Q82: A solution containing 10.0 g of an
Q83: The concentration of urea in a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents