What is the concentration (M) of HCl in a solution that is prepared by dissolving 25.5 g of HCl in 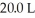 of water? (Assume volume does not change after adding HCl.)
of water? (Assume volume does not change after adding HCl.)
A) 1.28
B) 0.0350
C) 35.0
D) 3.50 × 10-5
E) 14.0
Correct Answer:
Verified
Q67: The molarity of urea (MW = 60.0
Q68: A solution at Q69: A solution is prepared by dissolving 24.7 Q70: A 30.0 g sample of potassium nitrate Q71: The concentration of urea (MW = 60.0 Q73: A solution is prepared by dissolving 15.8 Q74: The solubility of MnSO4 monohydrate in water Q75: The vapor pressure of pure ethanol at Q76: What is the mole fraction of HCl Q77: A solution is prepared by dissolving 15.0![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents