George is making spaghetti for dinner.He places 4.01 kg of water in a pan and brings it to a boil.Before adding the pasta,he adds 58 g of table salt (NaCl) to the water and again brings it to a boil.The temperature of the salty,boiling water is ________°C.(Assume 100% ionization of NaCl.) Assume a pressure of 1.00 atm and negligible evaporation of water.Kb for water is 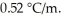
A) 99.87
B) 100.26
C) 100.13
D) 99.74
E) 100.00
Correct Answer:
Verified
Q86: The vapor pressure of pure water at
Q87: What is the freezing point (°C)of a
Q88: Which one of the following substances would
Q89: A solution is prepared by dissolving 0.60
Q90: A mixture containing 41.0 g of an
Q92: Determine the freezing point (°C)of a 0.015
Q93: What is the solubility concentration (M)of nitrogen
Q94: A saturated solution _.
A)contains dissolved solute in
Q95: A solution contains 39% phosphoric acid by
Q96: The solubility of oxygen gas in water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents